What is a Medical Device?
What is a Medical Device?
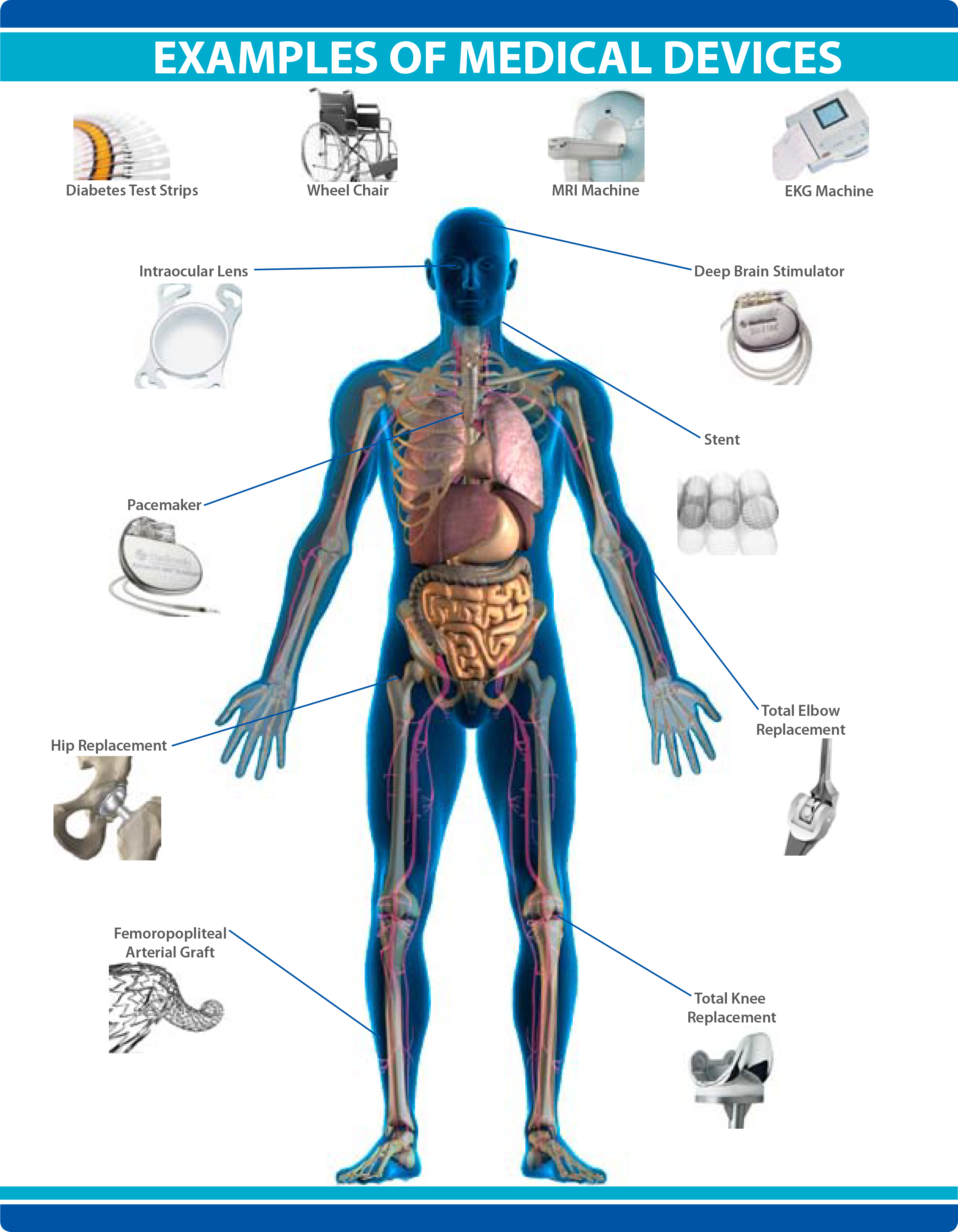
A medical device is an instrument, apparatus, implant, machine, tool, in vitro reagent, or similar article that is to diagnose, prevent, mitigate, treat, or cure disease or other conditions, and, unlike a pharmaceutical or biologic, achieves its purpose by physical, structural, or mechanical action but not through chemical or metabolic action within or on the body.
The US Food and Drug Administration (FDA) reviews medical technology according to the risk to the patient, with higher risk products requiring more clinical evidence than lower risk products. FDA allows on the US market only those products that have met its requirements.
FDA groups medical devices into three categories based on risk:
- Class I products are low risk and do not require submission of data or information to FDA.
- Class II products pose a moderate risk and are cleared via 510(k).
- Class III products are high risk, innovative products requiring a Premarket Approval Application.